

Fix N Fuse
TLIF cage

Fix N Fuse
ACIF cage
TLIF
Description
Fix N Fuse TLIF cage is intended to replace lumbar intervertebral discs and to fuse adjacent vertebral bodies at vertebral levels L2 to S1 following unilateral transforaminal (TLIF) discectomy for reduction and stabilization of the lumbar spine.
Manufacturing technology
Additive, 3D printing.
Material
Medical grade titanium alloy (Ti 6Al 4V ELI), which complies with the ISO 5832-3 standard.
Product overview
-
Anatomical design shaped for proper implant fit and smooth insertion.
-
Lordotized design to restore the natural spine lordotic curve.
-
Optimized implant / endplate contact surface to reduce the risk of subsidence, and to increase the stabilization of the pathologically unstable segment.
-
Biocompatible titanium alloy material with advantageous bioactivity.
-
Cavities and holes promoting ossification:
-
large central graft cavity,
-
additional 0.8mm holes through the endplates,
-
A-P holes and windows to promote the most complete ossification, and aid in visualization of fusion.
-
-
Toothed superior and inferior surfaces for increased primary stability.
-
Unique „sand timer” inserter connection for a tight fit and stepless, smooth insertion.
-
Self-distracting bullet nose facilitates insertion.
-
Clearly visible size marking which engraved in the material.
-
Products are provided in NON-STERILE condition. Must be cleaned and steam-sterilized prior to surgical use!





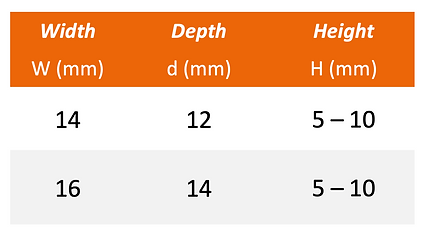
ACIF
Description
Fix N Fuse ACIF cage is intended to replace cervical intervertebral discs and to fuse adjacent vertebral bodies at vertebral levels C2 to T1 following anterior cervical discectomy.
Manufacturing technology
Additive, 3D printing.
Material
Medical grade titanium alloy (Ti 6Al 4V ELI), which complies with the ISO 5832-3 standard.
Product overview
-
Anatomical design shaped for proper implant fit:
curved superior and wedge inferior surface. -
Optimized implant / endplate contact surface to reduce the risk of subsidence, and to increase the stabilization of the pathologically unstable segment.
-
Biocompatible titanium alloy material with advantageous bioactivity.
-
Cavities and holes promoting ossification :
-
large central graft cavity,
-
additional 0.8mm holes through the endplates,
-
large lateral windows to promote the most complete ossification, and aid in visualization of fusion.
-
-
Toothed superior and inferior surfaces for increased primary stability.
-
Threaded inserter connection for rigid inserter-to-cage connection.
-
Concave posterior wall.
-
Through A-P hole for better visualization.
-
Clearly visible size marking which engraved in the material.
-
Products are provided in NON-STERILE condition. Must be cleaned and steam-sterilized prior to surgical use!
